Lithium-ion batteries are rechargeable batteries installed in devices such as smartphones and laptops that we use every day. The prototype of the battery was invented at the end of the 18th century, and it has developed over more than 200 years. Lithium-ion batteries are one of the latest types of batteries born in the process of battery development.
1. Characteristics of lithium-ion batteries
Batteries are classified into “primary batteries” which are dry batteries that can only be used once, and “secondary batteries,” which can be recharged multiple times. Lithium-ion batteries are rechargeable secondary batteries that are not only smaller and lighter in size than other types of batteries but also can store a higher amount of electrical energy.
2. How lithium-ion batteries produce electricity
In addition to lithium-ion batteries, various other types of batteries actually work on the same basic principle of producing electricity.
The battery has a favorable electrode (favorable electrode) and an unfavorable electrode (adverse electrode) made from steel products. A compound (electrolyte) that performs power via ions is filled up between the positive and negative electrodes. The steel electrode is thawed by the electrolyte and split right into ions and electrons. The electrons relocate from the unfavorable electrode to the positive electrode to create a current, and power is generated. In a secondary battery, electrons are saved in the negative electrode by billing prior to starting to make use of the battery. When the battery is used, the saved electrons transfer to the positive electrode to generate electrical energy.
Lithium-ion batteries use a lithium-containing metal compound in the positive electrode in advance, and the negative electrode uses carbon (graphite) that can absorb and store lithium. Through this structure, electricity can be generated without the need for the electrolyte to melt the electrodes like traditional batteries, thereby slowing down the aging of the battery itself. Not only can more electricity be saved, but the number of charge and discharge times can also be increased. In addition, lithium is a very small and light substance, which enables batteries to have various advantages such as being compact and lightweight.
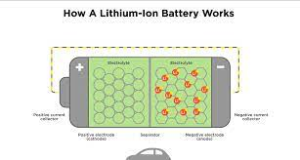
How lithium-ion batteries generate electricity
3. Are there various types of lithium-ion batteries?
Lithium-ion batteries are divided into several categories based on the metal materials used in the positive electrode. The original metal material used in the positive electrode of lithium-ion batteries was cobalt. However, the output of cobalt is almost as small as that of lithium. It is also a rare metal, and its manufacturing cost is high. Therefore, materials with low environmental impact, such as manganese, nickel, iron, and other metals, have been used. Lithium-ion batteries are classified according to the materials used. Let’s take a look at the characteristics of each type.
Types and characteristics of lithium-ion batteries
Cobalt-based lithium-ion battery
The positive electrode in lithium-ion batteries typically consists of lithium cobalt oxide. This material is relatively simple to produce and utilize, which made it the preferred choice for the first mass-produced lithium-ion batteries. However, since cobalt is a scarce and costly metal, it is rarely used in automotive applications.

Manganese lithium-ion battery
The positive electrode in lithium manganate is advantageous because it has a voltage comparable to cobalt-based lithium-ion batteries and is cost-effective to produce. However, a drawback is that there is a risk of manganese seeping into the electrolyte. At the same time, the battery is being charged or discharged, which can result in a reduced battery lifespan.
Iron phosphate lithium-ion battery
Lithium iron phosphate is used as the positive electrode. The advantage of iron phosphate-based lithium-ion batteries is that even the internal heating structure is difficult to damage, is highly safe, and uses iron as raw material, so its manufacturing cost is lower than that of manganese-based batteries. But, the voltage is lower than other lithium-ion batteries.
Ternary lithium-ion battery
Ternary lithium-ion batteries are batteries made of three materials: cobalt, nickel, and manganese in order to reduce the amount of cobalt. Nowadays, most ternary lithium-ion batteries contain a higher percentage of nickel. Despite having a slightly lower voltage compared to the cobalt and manganese series, it has the advantage of reducing production expenses. Nonetheless, the synthesis and preparation processes for each material are challenging, and their stability could be more optimal. As a result, there are still unresolved concerns regarding their suitability as practical materials.
4. What is the distinction between lead-acid batteries and lithium-ion batteries?
In addition to lithium-ion batteries, there are several types of rechargeable batteries. Among them, lead-acid batteries have a long history and have been used for more than 100 years. Even with the development of new batteries, such as lithium-ion batteries, they are still used as automobile batteries.
The distinctions between lead-acid batteries and lithium-ion batteries
Lead-acid batteries use lead as both positive and negative electrode materials, making them very cheap to manufacture compared to lithium-ion batteries. But because lead is heavier than other metals, the battery itself is also heavier. In addition, the maximum voltage can only reach 2V, and large self-discharge is also a shortcoming of lead-acid batteries.
The reason why lead-acid batteries are still used today
Although lead-acid batteries have these shortcomings, the reason why car batteries have yet to be replaced by high-performance secondary batteries such as lithium-ion batteries is that they are cheap, the technology is basically mature, and the reliability is high. Cars make full use of the characteristics of lead-acid batteries and establish a recycling system. If you switch to a new type of battery, the circuit design needs to be modified. Based on the current situation that lead-acid batteries are still sufficient to function, manufacturers want to avoid paying extra costs.
Even so, lithium-ion batteries are already used as secondary batteries for driving the engines of electric vehicles, electric-gas hybrid vehicles, etc., and lead-acid batteries will no longer be used in cars in the future.

Supplier
TRUNNANO is a supplier of lithium-ion batteries’ raw materials with over 12 years experience in nano-building energy conservation and nanotechnology development. It accepts payment via Credit Card, T/T, West Union and Paypal. Trunnano will ship the goods to customers overseas through FedEx, DHL, by air, or by sea. If you are looking for high-quality lithium-ion batteries’ raw materials, please feel free to contact us and send an inquiry.